In-use stability studies
Assessing the impact of product-handling by in-use stability studies

A drug substance not only needs to be stable during product, transport, and storage, but needs to withstand the handling procedures during administration. In a clinical setting, a drug product may be diluted and exposed to different surface materials including as tubings, infusion bags, pumps, closed system drug transfer devices, and filters before it is finally administered to the patient. In-use stability studies aim to assess the effect of such product handling on the stability and integrity of the drug product and thus therapeutic efficacy and safety.
Factors influencing the in-use stability
During an in-use stability study, we assess a variety of factors, including the effect of dilution and the dilution medium, potential adsorption to surface materials, shear forces during application, variations in extractable volume and reconstitution, formation or introduction of aggregates and (sub)visible particles, and more. While individually designing each in-use stability study, we commonly include a selection of the most promising drug product candidates using fresh as well as aged material and assess the stability.
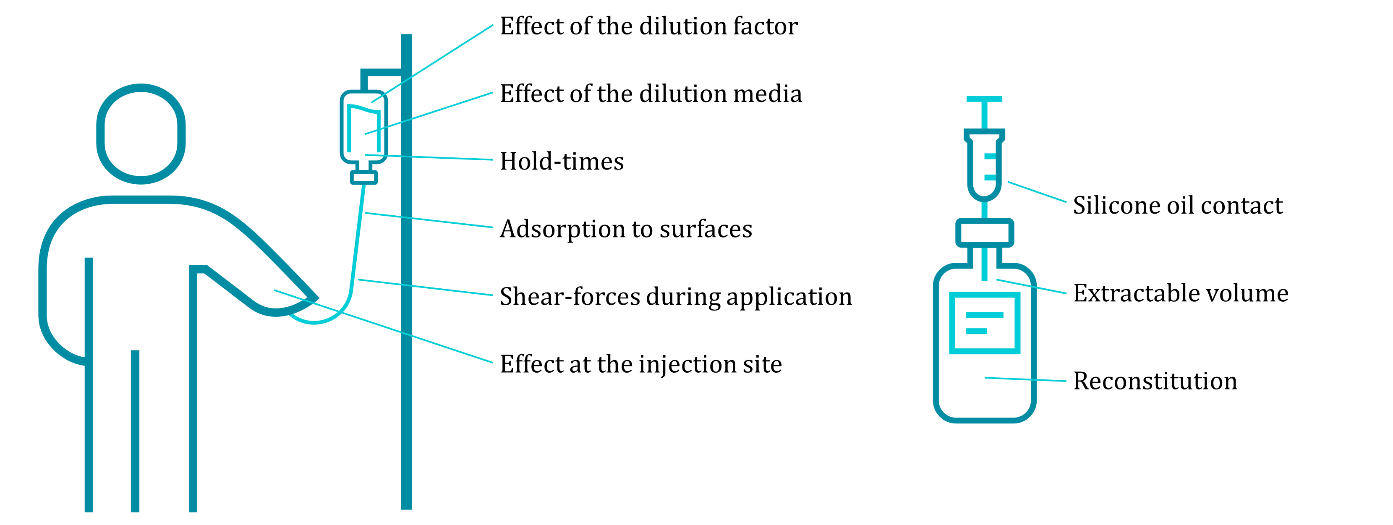
In-use stability under R&D and GMP.
Coriolis offers in-use stability studies in various quality levels, which can be tailored your needs.
- R&D level: Full flexibility following our internal Good Research Practice Guidelines
- Enhanced R&D level: Controlled environment and qualified equipment with full R&D flexibility
- GMP level: GMP environment with full QA involvement
Do you have any questions?
Our experts are happy to discuss your questions and inquiries related to in-use stability studies!
In-use stability studies tailored to your drug product
We offer in-use stability studies will be tailored to your specific drug product, development phase, clinical material and application procedures, and is backed by many years of experience with similar drug products. Click on a product type to explore our entire portfolio of specialized development and analytical services.
Our quality standards
Quality & biosafety level of this service
We provide all our services with the highest quality standards. Each project is carried out by experienced scientists and every report or data presentation is comprehensively checked by a scientific reviewer. We offer this service with the following quality and biosafety level:
Our knowledge
Our know-how related to in-use stability studies
Coriolis is a science-driven service provider. We perform internal research projects with academic and industrial partners to develop new technologies and create in-depth knowledge, which directly benefits our client projects. Our internal Unit Science & Technology, hosting PhD candidates and Postdocs, also actively contributes to the scientific community with numerous peer-reviewed publications each year. Explore our latest publications, articles, and webinars:
Let's talk!
Find out how you can benefit from a tailored in-use stability study from the scientific expert.
Other services
Our other services
Coriolis provides a wide range of science-driven and tailor-made solutions for research, development, and analytical challenges. Explore our other services and learn more about how we can support your drug development program. Together, we can make future therapies available to patients and improve the quality of life for humankind.
Contact us
Contact us
Please contact Dr. Matthias Lucke for inquiries related to in-use stability studies
Phone: +49 89 41 77 60 – 253
Mail: matthias.lucke@coriolis-pharma.com