Lyophilization Process Development at Light-Speed

See the Big Picture - A research summary
Continuous technological advancements and scientific discoveries have allowed pharmaceutical researchers to develop more and more complex drug compounds to address many challenging medical needs. For example, the utilization of antibodies and hormones allowed the treatment of autoimmune diseases. Lipid nanoparticles enabled the delivery of mRNA vaccination against Covid-19 and the rapidly growing class of advance therapy medicinal products (ATMPs) now also expands the use of viral vectors and living cells as therapeutic compounds. The growing complexity of these compounds challenges scientists to obtain formulations conditions, in which the active pharmaceutical ingredient (API) is stable and functional for the desired storage period.
A liquid formulation is the generally preferred format because it is user-friendly and often fast and relatively inexpensive to produce. But for complex molecules, a liquid format may not deliver the desired stability profile since water serves as a central mediator for most degradation pathways. Simply freezing the formulation during storage may circumvent stability issues for some compounds, but dramatically increases transport and storage efforts. Therefore, removing water from the formulation by freeze-drying can significantly improve the stability profile and the shelf life of a drug product. That is why we see an increase in the number of lyophilized drug products being developed. Freeze-drying, however, comes with its own challenges: the development of a freeze-drying process can be complex and labor-intense; the final process may be time-consuming and expensive; and trained personnel is often required for reconstitution and subsequent administration.
Challenges during lyophilization process development
Freeze-drying is usually done in three steps: 1) freezing, 2) primary drying and 3) secondary drying, as outlined in Figure 1. During step 1, the drug product is frozen via uncontrolled or controlled nucleation (Read more about controlled nucleation in our journal publication from 2018).
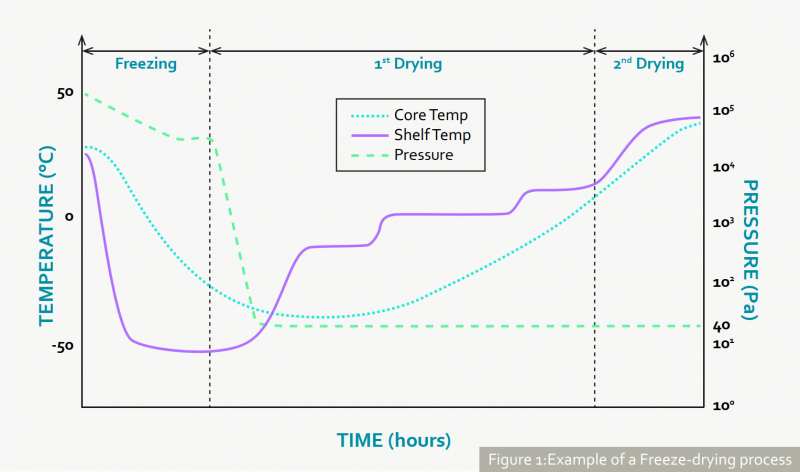
During step 2, a vacuum is applied and the shelfs holding the vials are set to an elevated temperature, resulting in a sublimation of most of the water inside the drug product. During step 3, the drug product is further dried until the residual moisture is reached, at which the product shows its highest stability. Step 2 – primary drying – is the longest and most challenging of the freeze-drying process. Developers aim to complete primary drying in the shortest possible time to reduce the overall process duration. However, if an excessive energy is supplied to the product during this phase, a partial or total change in the structure of the dried material (called collapse) can occur, negatively affecting the product stability. Critical parameters during this step are the heat transfer coefficient (KV), which describes the energy transfer from shelf to vial, and the product resistance (RP), which considers the effect of formulation composition and freezing conditions on the sublimation. Knowing these parameters as a function of shelf fluid temperature (Tfluid) and chamber pressure (PC) allows scientists to define a design space: a combination of input variables and process parameters (including Tfluid and PC) that provide the product in desired quality.
Building a design space usually requires an extensive experimental campaign. The most accurate technique for determining KV and RP is the gravimetric method: Individual vials are weighed before and after the experiment to determine the amount of sublimed solvent. This technique is, however, invasive and requires the process to be stopped in order to obtain reliable readouts during primary drying. Another technique, which is very frequently used, combines the pressure rise test (PRT) with tunable diode laser absorption spectroscopy (TDLAS) and can monitor an ongoing process in “real-time”. Unfortunately, this technique is not applicable to every equipment type and it provides only an average KV for all vials in the freeze-dryer without the possibility for differentiation of vial location (e.g., center or edge vials). Ideally a fast, non-invasive technique applicable to any sort of freeze-dryer, would be necessary to enable the characterization of a given lyophilization process.
A fast and universally applicable method to determine the design space
Researchers at Coriolis have been studying the application of a modern process analytical tool called heat flux sensor (HFS) to facilitate the development of a robust and quick primary drying. This sensor is place between the shelf and the vials in order to monitor the transfer of energy from the shelf to the product, the product temperature and critical end points during freeze-drying (Read more about heat flux sensors in our journal publication from 2017). While this sensor has been applied during process development for some years now, the research group around lead scientists Marco Carfagna developed, for the first time, a design space through the application of an HFS with only one experimental run (access the full text publication). The process parameters generated by HFS were compared to the most accurate, but time-consuming and invasive, gravimetric method. Then, the ability to generate a design space was tested and verified by transferring an entire process to a second freeze-dryer using a design space generated from HFS data. Product analysis confirmed the reliability of the created design space in the case study. “The HFS approach can substantially accelerate process development by determining all relevant information for the development and transfer of a primary prying step, minimizing time, energy and material consumption.” said Marco Carfagna.
While this proof of concept is a big step forward, the researchers at Coriolis are currently applying the HFS approach to a variety of bio-pharmaceutically relevant formulations, including amorphous and crystalline formulations as well high protein concentration formulations. The final goal of the research group is to assess, if HFS can be a routine in-line PAT tool to design and control the cycle without any limitations for formulation type and/or freezing protocol.