Analytical ultracentrifugation (SV-AUC / SE-AUC)
Analytical Ultracentrifugation

Analytical Ultracentrifugation (AUC)has long been recognized as a powerful technique, even a gold standard, for the detailed study of macromolecules, including proteins, nucleic acids, viral vectors and nanoparticles. Its ability to provide precise information on aggregation, molecule size, shape and interactions has made it indispensable in the field of biophysical characterization.
AUC – or more precisely the sedimentation velocity (SV-AUC) and sedimentation equilibrium (SE-AUC) method – is based on a simple principle: when (macro)molecules in solution are subject to a centrifugal force, they begin to settle at a certain velocity. With a mathematical correlation between sedimentation behavior and hydrodynamic properties, scientists can determine many critical parameters of a drug substance or drug product for a variety of biopharmaceutical applications from analyzing small peptides to investigating macromolecular interactions.
Coriolis is a globally operating service provider and one of the world leaders for SV-AUC and SE-AUC services. We operate the latest AUC technology equipped with a best-in-class selection of detection methods. Our dedicated AUC team is highly experienced in a number of (bio)pharmaceutical applications and has developed a set of robust generic methods, ready for application many samples and applications. Above that, our scientists love to push the limits of the AUC technology tailor-made methods for more challenging projects.
Our AUC services cover a large variety of applications for peptides, proteins, viruses, nanomedicines and more. We work with samples up to biosafety level S2. We offer AUC under various conditions to support our clients in all phases of product development through to market approval.
Applications of AUC
Analytical ultracentrifugation is a highly versatile analytical method and brings valuable benefits during the research and development of (bio)pharmaceutical drug products. Coriolis is one of the world leaders for SV-AUC and SE-AUC services and offers AUC services for a variety of (bio)pharmaceutical applications:
Analytical ultracentrifugation is highly suited for the accurate determination of important parameters of a drug substance, including molecular weight Mw, diffusion coefficient and sedimentation coefficient. As a first principle method, AUC does not require reference standards for such determination. Particularly SE-AUC is a very powerful method delivering the most accurate information on the (concentration-dependent) apparent molecular weight of the species in solution.
SV-AUC determines the content of low- and high molecular-weight species without any change in solution condition (if formulation components do not interfere with the detection). Thus, SV-AUC is the ultimate standard for determining the content of fragments, oligomers, aggregates and even nanoparticles in (bio)pharmaceutical solutions.
The size range of AAVs (around 25 nm) is ideal for the analytical characterization by SV-AUC, particularly the quantification of empty vs. full capsids.
HP-SEC and AF4 commonly used methods for determining fragments, oligomers, and aggregate content in (bio)pharmaceutical samples. While both techniques have numerous advantages, they also have a key fundamental limitation: in most cases a change in solution condition (i.e., mobile phase) is required. This may alter the ‘true’ aggregate population in a given sample prior to detection. SV-AUC can detect aggregates without any change in solution condition (if formulation components do not interfere with the detection). Thus, SV-AUC is often performed as an orthogonal method during the development of HP-SEC and AF4 to ensure the validity of their results.
SV- and SE-AUC are very powerful methods for the characterization of nanoparticulate species up to 200 nm, including viruses, virus-like particles, nanoparticles, liposomes, drug delivery systems and particle-based vaccines.
While many analytical techniques are unable to directly measure highly concentrated samples (such as >100 mg/ml antibody solutions), SV- and SE-AUC can be applied for highly concentrated samples, e.g., during formulation screening, without the need for sample dilution. By applying interference optics, highly concentrated sample can often be analyzed by AUC 'as-is', enabling an unaltered view on the sample’s properties.
Analytical characterization of viruses, such as retroviruses, lentiviruses, and adenoviruses, is possible by SV- and SE-AUC for sizes up to 200 nm. We offer stability testing of viruses by AUC as a stand-alone service or as part of a virus formulation development project. Our AUC experts can also combine the power of AUC with orthogonal method, such as AF4, for enhanced insights.
As a first principle method, AUC does not rely on (internal) standards and is thus well suited for the reliable determining of interaction parameters (KD, Stoichiometry, B22). It does so without the need for protein binding or immobilization (as needed for Surface Plasmon Resonance measurements).
Coriolis applies the latest in AUC technology (Beckman Optima III) and a high-speed sample-rotor. This allows our scientists to apply centrifugal forces of 290,000 g, which can sediment peptides down to 1 kDa and, thus, enable their analytical characterization.
SE-AUC is a very powerful technique delivering the most accurate information on the (concentration-dependent) apparent molecular weight of the species in solution.
We are proud to offer fluorescence detection as an option for AUC analysis. This allows for an selective analysis of labeled molecules in a sample mixture, excluding interferences from other (unlabeled) species.
Coriolis employs AUC instruments with fluorescence detection. This enables in-depth analysis of protein-protein interactions with fluorescent tracers. With this, a multitude of application scenarios and assays tailored to specific research questions becomes available.
AUC measures samples undiluted, does not have a stationary phase and utilizes high-sensitivity UV or fluorescence detection. Thus, analytical characterization of low-concentration samples (e.g., diluted virus preparations) is possible by SV- and SE-AUC.
The benefits of AUC by Coriolis
Coriolis offers you the scientific excellence of a world leader with high-quality AUC services that bring real benefits to your product development. Learn why Coriolis is your best choice when it comes to AUC.
Our AUC facilities meet even the highest standards. We operate the most modern AUC instruments - the Beckman Optima - and offer high-speed as well as high-throughput sample rotor option to best serve your needs. Our broad range of detector options includes UV-absorption, Rayleigh interference (RI) and fluorescence and enables all application examples outlined above.
Within our large portfolio of 100+ different analytical techniques, we offer many orthogonal methods to AUC including SEC-MALS, AF4-MALS and surface plasmon resonance (SPR). All analytics are performed in-house, so we can easily incorporate them for comparison or verification.
We run even the most demanding data processing (SedFit & UltraScan) on our in-house computer cluster, without relying on external / third-party services. Your data will be handled, processed, and stored securely and confidentially with us.
Our dedicated AUC team handles many challenging projects every year and has experience with a large variety of (bio)pharmaceutical drug products. Supported by our distinguished scientific advisory board, our scientists are eager to push the limits of the AUC technique.
We offer more than just high-quality results. We additionally provide our clients with the required interpretation and scientific advice to see the data in context of the overall scope of analysis and/or development.
AUC is often considered a low-throughput technique. But with our instrumentation and expertise, we can process up to 14 samples per run. This is not much different to the throughput of many HPLC sequences.
We offer a set of robust generic methods developed with in-house expertise and ready for many samples and applications. Above that, our AUC scientists can develop tailor-made methods for more challenging projects and perform product-specific method qualification, if desired.
With five (5) AUC systems and a dedicated AUC expert team, we offer many resources for AUC projects. This allows us to accommodate most projects within quick response times.
AUC is a valuable technique for the analysis of viral vectors, virus formulations, GMOs, and other biological agents. Thus, we offer AUC services also for samples up to biosafety level 2 / S2.

Our AUC equipment
Coriolis is one of the world leaders for SV-AUC and SE-AUC services and we operate the latest AUC technology equipped with a best-in-class selection of detection methods. Coriolis processes and stores all data in-house by using our own AUC computer cluster. Neither your samples nor your data will be sent to third parties.
Our AUC facilities include:
5 AUC instruments of various types
- 2x Beckman Optima AUC
- 2x Beckman XL-I
- Beckman XL-A
3 Detection methods
- UV-Absorbance
- Rayleigh interference (RI)
- Fluorescence
2 Data processing methods on dedicated in-house computer cluster!
- SedFit
- UltraScan
Laboratory facilities up to BioSafety Level S2
Principles of AUC
Analytical ultracentrifugation is a powerful technique for the analysis of (bio)pharmaceuticals. Below, we will introduce you into the two principle operation modes of AUC: the more common sedimentation velocity (SV-AUC) method and the sedimentation equilibrium (SE-AUC) method for accurate molecular weight information.
Sedimentation velocity method (SV-AUC)
When macromolecules in solution are subjected to a centrifugal force, they will begin to settle at a certain velocity. This sedimentation velocity depends on the instrument settings (mainly the speed of rotation), the solution / formulation (density, viscosity) and – of course –the macromolecule itself (mass, density, and shape).
In Figure 1, a cross section of an SV-AUC center piece with two sectors is shown. One sector is usually filled with a blank or placebo, matching the macromolecule solution / formulation. The other sector is filled with the molecule sample. When a centrifugal force is applied, macromolecules move towards the bottom of the cell and are thus depleted from the solution at the top. This way, a boundary is formed between the solution containing the macromolecules and solution depleted of molecules.
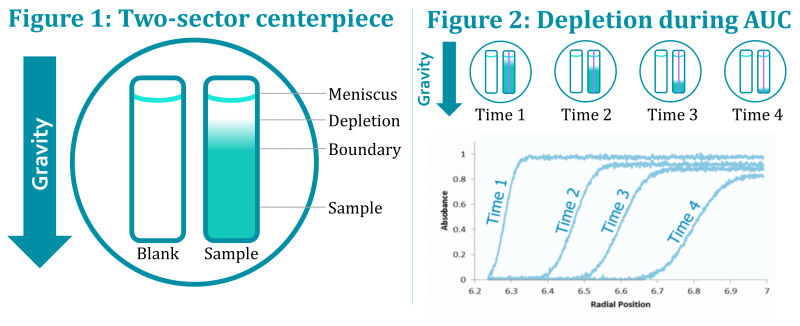
During an SV-AUC run, the time-dependent concentration profile of the macromolecule along the entire length of the centerpiece is measured using one or more of the following detection methods: UV absorbance, Rayleigh interference or fluorescence detection. In Figure 2, you can see how this signal changes over time, reflecting the depletion of the macromolecule during the run.
The so obtained time-dependent concentration profile is then processed by computer algorithms that fit these data to appropriate mathematical models. Advanced algorithms – such as those performed by the UltraScan software – require a high-performance computer cluster.
During analysis, the travel speed of the boundary provides information about the size, shape, and mass of the macromolecules. The shape of the boundary layer provides quantitative information about the distribution of differently sized macromolecules in solution (e.g., amount of dimers / aggregates).
With specifically designed rotors and modern AUC systems, centrifugal forces of up to 290,000 g can be applied. This allows for the sedimentation and, thus, characterization of molecules with a mass as low as 1 kDa (e.g., peptides).

Sedimentation equilibrium method (SE-AUC)
SE-AUC uses the same physical principles and detection mechanisms as SV-AUC, but with a crucial difference: it is performed at a lower rotation speed where back-diffusion – from the bottom of the cell (high concentration) towards the top (low concentration) – becomes significant.
Over time, this back-diffusion reaches an equilibrium with the sedimentation movement and forms a stable concentration profile. For an extended period, the formation and stable position of this profile is recorded. This delivers most accurate information on the molecular weight of the species in solution because the molecule-shape is not influencing the results of SE-AUC as it is the case for HP-SEC, AF4 or SV-AUC.
Coriolis has used its instruments to successfully investigate molecules as large as MDa polymers and as small as 1-kDa peptides by this approach. SE-AUC is currently the gold standard method for determining molecular weights of macromolecules in their native buffer solution.
Contact us
Contact us
For enquiries related to analytical ultracentrifugation please contact Dr. Jörg Müller
Phone: +49 89 41 77 60 – 111
Mail: joerg.mueller@coriolis-pharma.com


