Coriolis Inside – Our Philosophy about Partnering
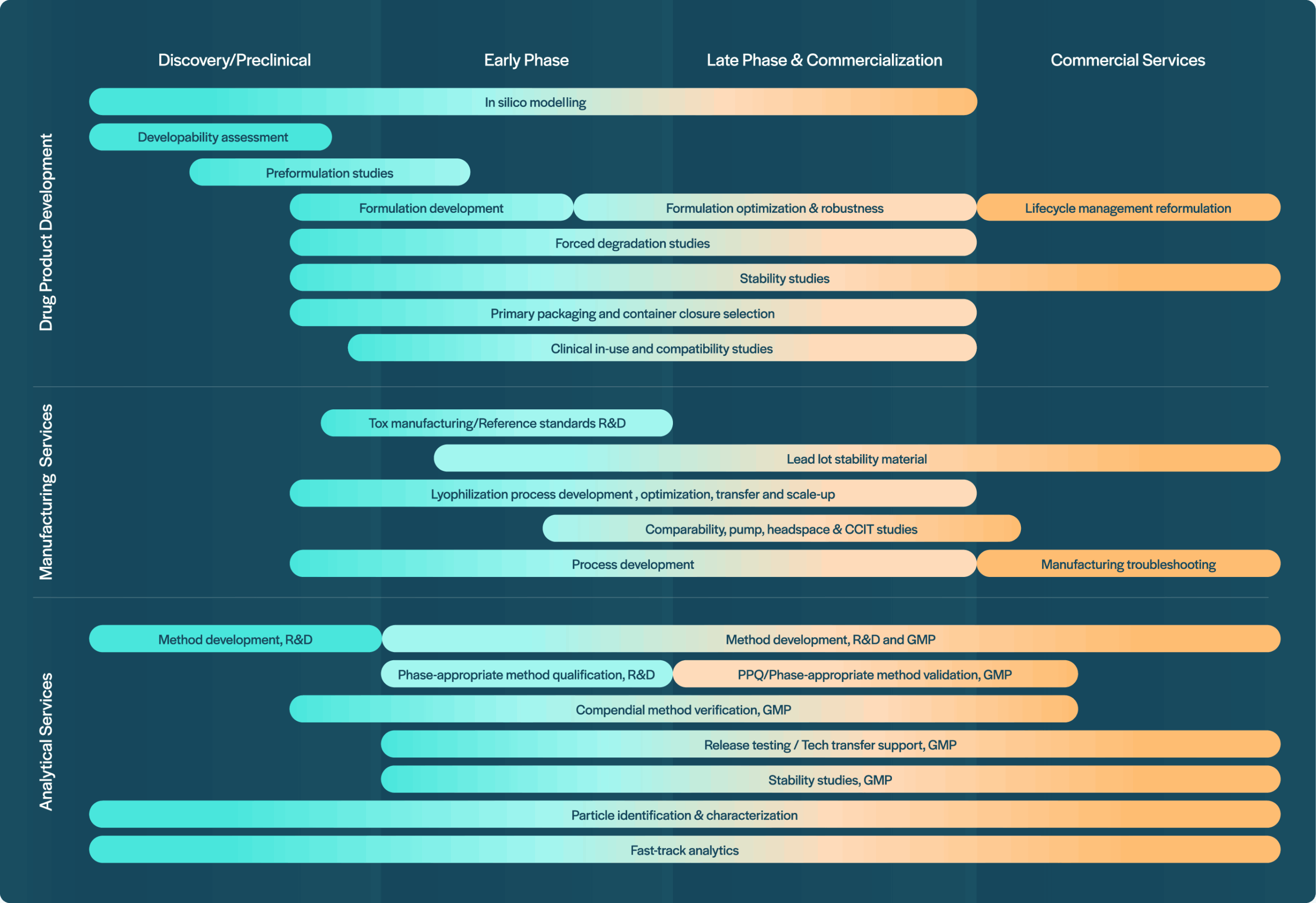
At Coriolis, we believe that scientific excellence, client-focused innovation, and strong partnerships are key to advancing complex biopharmaceutical programs efficiently and reliably. Across the entire value chain, we support the development of innovative biopharmaceutical products – from early formulation research to commercial manufacturing. We operate within a collaborative ecosystem, working closely but non-exclusively with strategic partners who share our values and help us shape the future of biopharma.
End-to-End Solutions with Strong Partnerships
Together with our partners in the field of drug substance manufacturing, fill/finish, and packaging, we provide end-to-end solutions – from early formulation through to commercial manufacturing – leveraging complementary expertise to accelerate the path to market. Coriolis Pharma contributes a unique combination of in silico and wet-lab formulation know-how, drug product development experience, scientific depth, and advanced analytical capabilities to all these collaborations.
Rentschler Biopharma is a leading contract development and manufacturing organization (CDMO) focused exclusively on client projects. The company offers process development and manufacturing of biopharmaceuticals, as well as related consulting activities, project management and regulatory support. Rentschler Biopharma’s high quality is proven by its long-standing experience and excellence as a solution partner for its clients. A high-level quality management system, a well-established operational excellence philosophy and advanced technologies ensure product quality and productivity at each development and manufacturing step. Rentschler Biopharma is a family-owned company with about 1,400 employees, headquartered in Laupheim, Germany, with operations in Milford, MA, USA. In 2024, the company joined the United Nations Global Compact, emphasizing Rentschler Biopharma’s focus on sustainability.
Founded in 1949, Stevanato Group (formerly known as OMPI) is a worldwide leading provider of primary packaging and delivery systems for injectable drugs. Backed by 75+ years of scientific expertise, along with its commitment to technical innovation and engineering excellence, Stevanato Group offers a unique integrated offering to biopharmaceutical companies to streamline the combination product development journey.
Talk to Our Experts or Request a Quote
Our expert team is ready to answer your questions and guide you to the services best suited to your program’s modality, stage and challenge. If your needs are well-defined, we’ll begin the quotation process.
