High Concentration Protein Formulation – Challenges and Solutions
Biopharmaceutical companies have increased their efforts in developing protein-based drug products with a high concentration. This article provides an overview on why a high concentration formulation is desirable, how to achieve a stable high concentration protein formulation and how to analyze them.
The article includes the following:
- Reasons to formulate proteins or peptides at high concentration
- Answer the question: When is a protein concentration considered as “high”?
- Challenges of formulating a protein at a high concentration
- Analytical characterization of a high-concentration protein formulation
- How to formulate and stabilize a protein at high concentration in a liquid formulation
- Benefits of an optimized liquid high-concentration protein formulation
This article focuses on high protein concentration products in the liquid state, as this is often the first choice for drug product developers. We will, thus, use the term high concentration liquid formulation (HCLF) and high concentration protein formulation (HCPF) as synonyms. Lyophilized high protein concentration products, usually developed as a back-up scenario or intermediate stage, are not discussed here. Their development, however, provides its own special challenges, such as process set-up for products with large solid content or long reconstitution times before the product can be administered.
Why formulate a drug product at a high concentration?
Let’s start with the obvious: increasing the concentration of the active pharmaceutical ingredient (e.g., a protein or peptide) reduces the required application volume to administer the same dose. A small application volume can be a huge advantage for patients, as it allows for a self-administration of the drug at home via a subcutaneous injection instead of having to go to the hospital for a lengthy intravenous infusion. A conventional subcutaneous injection has a volume smaller than 2 ml. Convenience through auto-injection devices is of particular importance for people suffering from chronic diseases such as asthma, dermatitis, colitis, or arthritis, who need to receive their drug regularly. Subcutaneous injection also allows for a less frequent dosing as it results in a slower systematic availability compared to intravenous application, which improves pharmacokinetic and pharmacodynamic (PK/PD) profiles. Thus, a high-concentration subcutaneous drug product is a valuable alternative to an intravenous application. Depending on the molecule and the dose requirements, other routes of administration may also require high protein concentrations, for example intravitreal administration, where tiny volumes of around 50 µl are usually applied.
A high-concentration subcutaneous drug product is a valuable alternative to an intravenous application.
High-concentration formulations are developed mainly for proteins and peptides, with monoclonal antibodies being the most prominent biopharmaceutical subtype. Exploring new application areas with novel drug products (new IND filing) may, in some cases, only be feasible with a high-concentration formulation. However, already marketed products are also frequently reformulated (and patented) at high concentrations to profit from the above-mentioned benefits.
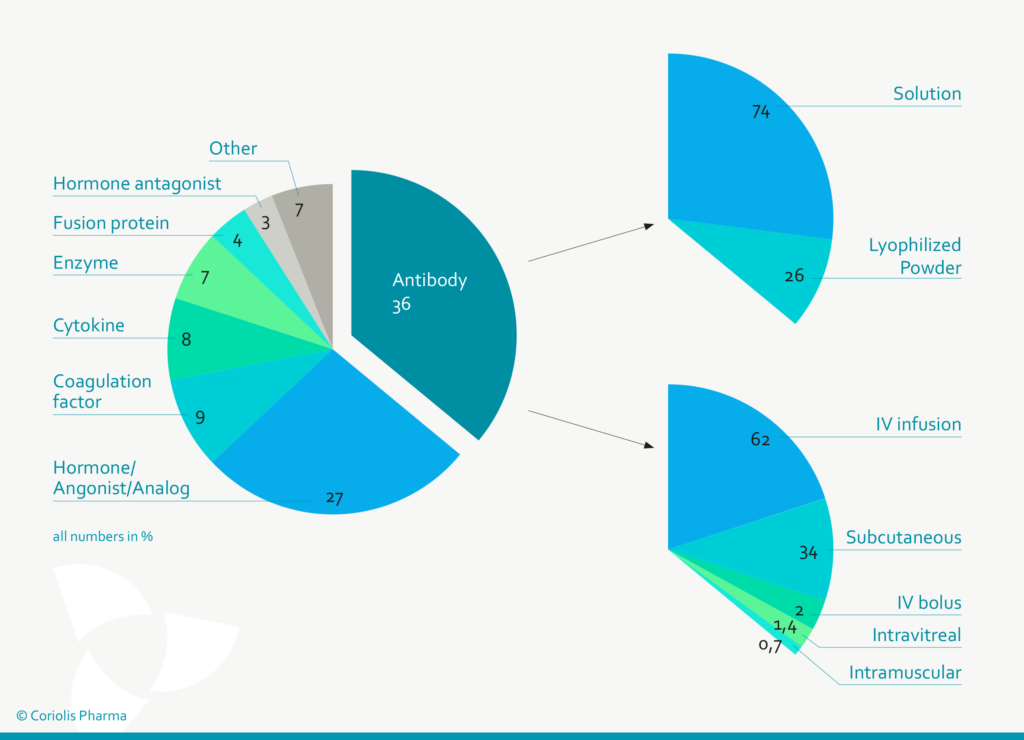
Figure 1: Antibodies are (with ~ 36%) the predominant class of protein therapeutic, of which 74% are formulated as liquid solutions and 34% are applied subcutaneously (Data from Gervasi et al. 2018 and Strickley et al. 2021).
When is a protein concentration considered as “high”?
A high concentration of a molecule is reached when its “solution properties” change, e.g., the thermodynamic solubility is reached, aggregation/precipitation occurs, or other phenomena start to appear, such as gel formation, phase separation, or increasing viscosity. These properties occur due to complex self-interaction and crowding effects, which polymers such as proteins exhibit at high concentrations, and which can impair processability and feasibility of injection (syringeability). Generally speaking, the term “high protein concentration” is a relative term and specific to every molecule or – to put in simple terms – a high concentration is where the trouble starts.
For monoclonal antibodies, a high concentration liquid formulation typically refers to protein concentrations above 50 mg/ml (frequently 100-150 mg/ml). For some peptides, a concentration at or below 10 mg/ml may already be considered high.
The challenges of formulating a protein at high concentration
Colloidal or other physical or chemical instabilities are of course a challenge for every drug product and need to be addressed through a dedicated formulation development. However, certain product attributes specific to high concentration liquid formulations pose very specific challenges to the formulation scientists and manufacturers. Here some important examples:
High viscosity and its implications
The most important fact about the viscosity of high protein concentrations is that their dynamic viscosity does not increase linearly with increasing concentration, but exponentially (see Figure 2). Hence, small changes in protein concentration may substantially impact the resulting viscosity of the drug product (and variability thereof). The dynamic viscosity also strongly depends on the product temperature and the shear stress applied. This has an influence on:
- the measurement conditions of analytical methods (as outlined later in this article)
- the manufacturing conditions, because of the different shear stresses applied in different manufacturing steps and
- storage of the bulk product
- the drug product characteristics during its shelf life, since the storage temperature likely differs from the temperature during application
- the final administration by the patient
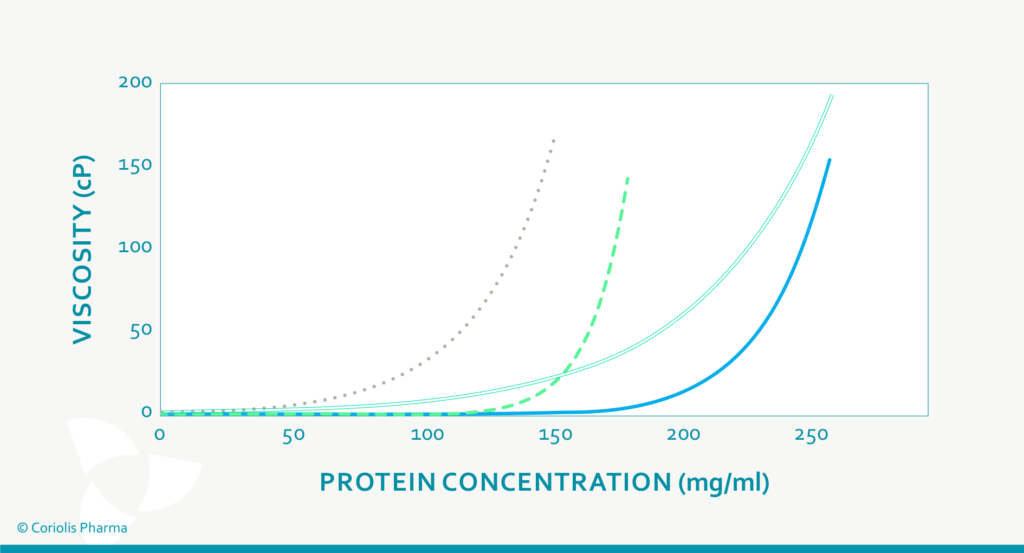
Figure 2: Viscosity of different protein (formulations) at increasing protein concentration (schematic drawing).
Chemistry, Manufacturing and Controls (CMC)
Some characteristics of high-concentration drug products can also be critical from a manufacturing point of view. For example, concentration and buffer exchange processes in the biopharmaceutical industry typically comprise an ultrafiltration/diafiltration (UF/DF) step. High material viscosities may result in extended process times (and hence production costs and stability issues) and in the worst case exceed system limitations, making production (via UF/DF) no longer feasible. In addition, the concentration of excipients may deviate from the expected concentration due to the Gibbs-Donnan effect – an accumulation of molecules at the filtration membrane. This is more pronounced at high concentrations and should be considered during development.
Fill and finish activities, including sterile filtration, may also be compromised by an unfavorable rheological behavior of the material due to a high protein concentration. Resulting elevated shear forces can also negatively impact product stability.
Syringability and the force required for injection
Prefilled syringes are currently the preferred primary packaging material for subcutaneously administered high concentration liquid formulations. They facilitate a convenient self-administration of drug product volumes between 0.2 – 2.0 ml and can be used in combination with auto-injectors. Prefilled syringes are available in various types (volumes and needle diameters) and materials (glass or plastic).
When developing a high-concentration drug product in prefilled syringes, it is crucial to keep the injection force – the force needed to expel the drug from the syringe – at acceptable limits. Elderly people, rheumatoid patients, or children need to potentially be able to self-administer the drug and, thus, a force below 25 N is usually desired. The injection force is thereby not only influenced by the drug product viscosity, but also by the syringe and needle type and dimension and liquid temperature. Also, the rheological behavior under varying shear stress (upon administration through the needle) will be more important than the viscosity value per se, which refers only to a single shear stress at a defined temperature. Empirical measurements of the syringeability are important in this context because most high concentration liquid formulations exhibit non-Newtonian flow behavior at high shear rates. The syringeability results are used to optimize the formulation but also to adjust the prefilled syringe configuration (e.g., adjust and define needle dimension).
A separate challenge, which applies to all injectables, arises from the siliconization of glass prefilled syringes, which facilitates plunger movement. Due to its hydrophobic nature, silicone oil can induce protein unfolding, aggregation or particle formation. As such effects could compromise drug efficacy and safety, they need to be monitored during stability studies over extended time periods. This requires an orthogonal set of analytical techniques for product and drug substance characterization.
Residual host cell proteins and polysorbate degradation
Host cell proteins (HCP) are an impurity originating from the production cell line. Even trace amounts can impair drug product stability and safety in various ways. One of them is the degradation of polysorbate, a frequently used protein stabilizer. Host cell proteins may contain enzymes that hydrolyze polysorbate. While host cell proteins are usually removed during downstream processing, a high concentration of the pharmaceutically active protein makes this task more challenging. Thus, formulation development for a polysorbate-containing high-concentration product should always include a polysorbate characterization to indicate the presence of residual host cell proteins.
Analytical characterization of high-concentration protein formulations
Stability-indicating analytical methods tailored to the respective research or clinical phase (phase-appropriate) are crucial for a successful drug product development. Without them, it would be impossible to tell the difference between a stable and an unstable formulation. The specific properties of a high-concentration protein formulation – namely, high viscosity, high refractive index and low sample volumes– require special attention when handling and analyzing these products. In general, the best approach and least manipulative way is to analyze samples undiluted because any sample preparation step may alter sample properties. For example, dilution could “dissolve” protein aggregates or even induce aggregation. Dilution itself might also be challenging for high-viscosity samples, particularly if the output parameters are volume-based (e.g., mg/ml).
However, many analytical techniques have a dynamic range too low to analyze high-concentration protein samples without prior dilution. This includes many light-based particle characterization methods, such as micro flow imaging (MFI) and light obscuration (PAMAS, HIAC), where a high refractive index of the sample decreases the detector sensitivity, and a high viscosity can reduce sampling accuracy. This also includes many spectroscopic techniques, which require sample dilution to avoid detector overloading.
A dedicated and science-driven method set-up for high protein concentration projects is critical!
Therefore, a portfolio of analytical methods capable of analyzing high-concentration drug products without further handling or dilution steps should be available for (sub)visible particle analysis, syringeability, protein content, optical density, turbidity, viscosity (rheological characterization), thermal stability, color, pH, conductivity, and osmolality. In cases where sample dilution is unavoidable, its influence on the results should be investigated e.g., during method development.
In general, a dedicated and science-driven method set-up for high protein concentration projects is critical to:
- define a robust and reliable sample preparation process (e.g., assessing the influence of dilution, careful selection of
- dilution media, gravimetric or volumetric dilutions)
- demonstrate the suitability of detection methods
- demonstrate specificity and stability-indicating potential of each method (what can be seen, what not?)
- characterize the sample from different “viewpoints” (completeness), by applying the right set of orthogonal methods.
How to formulate and stabilize a protein at high concentration
A formulation development for a high-concentration drug product has two goals: stabilize the protein and optimize viscosity. The latter is unique for high-concentration drug products. An optimized formulation is not only important for developmental work but also for later manufacturing (see CMC challenges above).
The first important question to be addressed is: How high can we go? The answer to that is the starting point – and in rare cases also end point – of all developmental work to follow. An up-concentration feasibility study answers this question and clarifies if certain manufacturing limits may be exceeded, e.g., viscosity/pressure limits for ultrafiltration/diafiltration. If so, alternative ways during the early stages of formulation development can be explored, e.g., discontinuous filtration.
During a formulation development, viscosity reducing excipients, such as certain amino acids or salts, are used to influence protein-protein interactions and to optimize drug product stability.
In general, the more concentrated a protein becomes, the more self-interaction will occur. This is due to van-der-Waals, ionic and/or aromatic interactions and commonly referred to as protein-protein interactions (PPI). Some molecules will tolerate or even promote these interactions more than others. They are called self-stabilizing proteins. The extend and type of protein-protein interaction will influence the solution viscosity as well as the colloidal and chemical stability of the protein. During formulation development, viscosity-reducing excipients, such as certain amino acids or salts, are used to influence protein-protein interactions and to optimize drug product stability. However, even with very favorable protein-protein interactions, every protein will eventually experience an increase in viscosity with increasing concentration due to an effect called crowding. This can rarely be omitted.
The pH of the formulation influences protein-protein interactions as well. Therefore, evaluating its impact on viscosity and stability is essential. The pH, defined in the target product profile (TPP) and subsequently targeted during the formulation strategy, should allow the protein itself to contribute to the overall formulation pH.
It becomes apparent, that each drug substance behaves differently under high concentration conditions, making a science- and knowledge-driven pre-selection and subsequent screening of excipients necessary. At Coriolis we benefit from our experience with more than 100 high concentration drug product projects (status 2021), making the development program more focused, knowledge-driven, and effective, and thereby increasing the chances of success.
The benefits of science-driven formulation development for high-concentration drug products
Developing a robust high-concentration protein formulation is a challenge. However, when an experienced formulation developer, such as Coriolis, works collaboratively with its client, the chances for success are high. The client contributes with his prior knowledge about the specific protein and delivers the target product profile (TPP). The formulation developer contributes with his knowledge gained from similar proteins, his experience in establishing suitable formulation strategies, a large portfolio of specialized analytical techniques and the expertise to establish suitable methods for drug product characterization.



