Particle Identification
Observing visible and subvisible particles can stagnate the drug development or the batch release process. Coriolis Pharma has the experience and the capacity to determine the identity and possible origin of particulate matter in your biopharmaceutical drug products—crucial for troubleshooting and root-cause analysis. Our experts can analyze your samples with minimal lead time and deliver results from the lab bench so you can resolve the underlying cause of particle formation.
Particle Identification Tailored to Your Needs
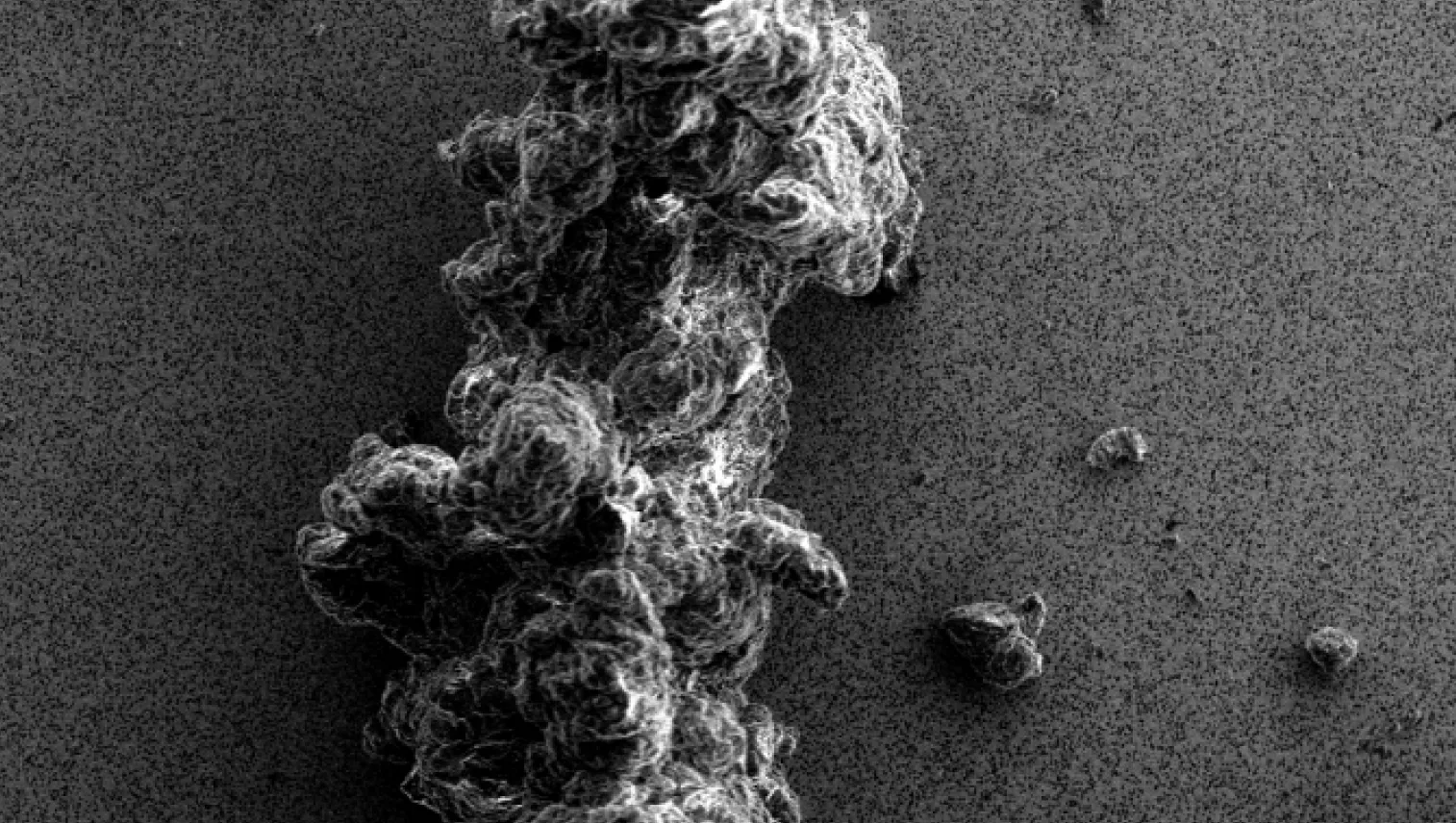
Particles in the visible and subvisible size range can appear in biopharmaceutical samples and drug products during all stages of development and even after market approval. The presence of particles can negatively influence the safety and efficacy of the therapy.
They can be introduced to a therapeutic in several different ways, including:
- Directly derived from the active pharmaceutical ingredient (API), for example, due to an insufficiently stable therapeutic protein (intrinsic particles)
- Be introduced by formulation excipients, for example, as degradation products or particulate impurities (inherent particles)
- Be generated by the production process, for example, during pumping or filtration steps (extrinsic particles)
Troubleshooting with Rapid Particle Identification
Determining the identity and possible origin of particulate matter in biopharmaceutical drug products is crucial for troubleshooting and root-cause analysis, and therefore, it is often very time sensitive. If required, Coriolis experts can analyze your samples within a matter of days with fast-track analytics so you can quickly resolve the underlying cause of particle formation.
Particle Identification Testing Methods
We can select the most suitable approach for your analytical challenge, whether following a standardized in-house workflow or a custom-tailored solution.
Analytical Method Development, Qualification and Validation
For common sample types, we can often apply standardized methods with little setup effort. However, when needed, our experienced analytical experts create or optimize custom methods tailored to your active pharmaceutical ingredient, product type and development phase.
Particle Identification for Your Drug Product
Coriolis Pharma’s deep expertise across many biologic modalities, combined with input from world-leading external advisors, offers the knowledge and experience needed to solve even the most complex formulation and drug development challenges.
Your Phase
Our expert team has the expertise and experience to guide your biopharmaceutical development program from preclinical and early-phase development to the market.
Particle Identification Resources

Press Releases
Coriolis Pharma Accelerates U.S. Expansion with Strategic Appointments in North Carolina
October 7, 2025
Particle Identification Frequently Asked Questions (FAQs)
-
Identifying visible and subvisible particles in drug products is essential for ensuring patient safety, product quality, and regulatory compliance. Particle contamination can delay batch release, trigger regulatory scrutiny, or compromise therapeutic efficacy.
-
Particles may be intrinsic (from unstable APIs), inherent (from excipients), or extrinsic (introduced during manufacturing, such as through pumping or filtration). Coriolis identifies all particle types using advanced analytical methods.
-
Coriolis employs a range of advanced techniques, including Fourier-transform infrared (FTIR) microscopy, Raman microscopy, light microscopy (LM), visual inspection, and scanning electron microscopy with energy-dispersive X-ray spectroscopy (SEM-EDS) to identify and characterize particles.
-
Yes. Coriolis offers rapid particle identification services with minimal lead times—often delivering results within days to support time-sensitive root-cause analysis and regulatory reporting.
-
Absolutely. Particles can arise during preclinical, early-phase, late-stage, and even post-market development. Coriolis supports particle identification throughout the product lifecycle, from preclinical studies to commercial batch troubleshooting.
-
By pinpointing the material composition and possible source of particles, Coriolis helps identify the root cause of contamination. This allows clients to implement corrective actions and prevent recurrence.
-
Coriolis supports a broad range of modalities including antibodies, peptides, therapeutic proteins, gene and cell therapies, viral vectors, nucleic acids, vaccines, and nanoparticulate delivery systems.
-
Coriolis will select the most appropriate method based on your formulation, particle characteristics, and development stage. Our team can provide custom analytical strategies to address even the most complex challenges.
-
Yes. Understanding the identity and origin of particles helps refine formulations and optimize processes to reduce particulate formation, improve stability, and ensure regulatory success.
Talk to Our Experts or Request a Quote
Our expert team is ready to answer your questions and guide you to the services best suited to your program’s modality, stage and challenge. If your needs are well-defined, we’ll begin the quotation process.


